Commercial
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.
Companies
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Small Sterilization Companies Poised To Meet EtO Emissions Goals On Time
Small sterilizers told Medtech Insight that they were ready for the EPA’s controversial EtO emissions rule, while community advocates expressed concerns.
Ultrahuman Expands Wearable Medtech Production Into US After $35M Funding Round
Firm operating in London, India and United Arab Emirates says its “Ultra Factory” will open in Indiana within the next six months with end-to-end production based on its operational facility in India.

Addition to Quest Alzheimer's Suite Looks For Biomarker P-Tau217
The test is being integrated into Quest’s AD-Detect portfolio for assessing the risk of Alzheimer’s. It is the third p-tau217 test to make news this month, after new breakthrough designations for Quanterix and Roche and Eli Lilly.

Alarming Rise Of Diabetes in Several US States, Study Shows
A study of diabetes rates across the US over four years reveals significant increases in the disease in many states. Tobias Oerum, diabetes advocate and cofounder of the company that conducted the study, discussed the data and some of the factors contributing to this troubling trend with Medtech Insight.
Deals and Financings
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

News We're Watching: LDT Final Rule Near Publication, Zimmer ROSA Used In Shoulder Surgery, $60M For Menopause Clinic
This week, the US FDA's proposed final rule on lab-developed tests cleared another hurdle on the path to release; Zimmer Biomet announced that its ROSA surgical robot had been used in shoulder replacement surgery for the first time; and a virtual menopause clinic closed out a $60M fundraising round.

Ultrahuman Expands Wearable Medtech Production Into US After $35M Funding Round
Firm operating in London, India and United Arab Emirates says its “Ultra Factory” will open in Indiana within the next six months with end-to-end production based on its operational facility in India.

Behavioral Health Company Two Chairs Secures $72M In Equity And Debt Infusion
Two Chairs, which uses algorithms to match the right therapists with patients, secured $72m in investment to expand its business.

Johnson & Johnson Poised To Lead Multiple Cardiovascular Markets With Shockwave Medical Acquisition
J&J’s acquisition of IVL device maker Shockwave Medical for $13.1bn sets J&J’s medtech arm solidly in a leadership position in multiple cardiovascular markets.
Market Access
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.
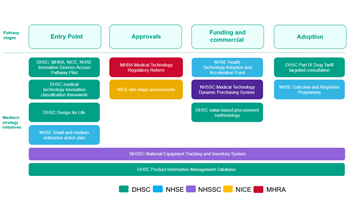
UK Medtech Strategy Sets Out Schedule Of Milestones To FY 2026
Fourteen months on from the release of its inaugural medtech strategy, the UK MedTech Directorate has laid firm foundations and reports progress on initiatives aimed at improving technology adoption. A schedule of ambitious future timelines has also been published.

Ekso's Exoskeleton Receives Medicare Coverage, Paves Way To Faster Rehabilitation
Ekso Bionics’ exoskeleton received a final payment determination from the Centers for Medicare and Medicaid Services, paving the way to faster rehabilitation for more patients.

News We’re Watching: Lung Preservation System Goes National, De Novo For MMI, Brazil’s ANVISA To Recognize Overseas Approvals
This week, Paragonix announced that its BAROguard lung preservation system is now available throughout the US; Brazil planned to leverage some foreign device approvals; Medical Microinstruments’ Symani Surgical System won de novo clearance; and the FDA updated its safety warnings for Essure and certain plastic syringes.

FDA Leader Looks To International Effort On Pediatric Device Development
Collaboration with Japan and Europe could help ease the development path for pediatric devices, cardiovascular device office director Bram Zuckerman said at a recent conference. Zuckerman also spoke on other cardiac device priorities at the FDA.
Market Intelligence
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

German IVDs At The Turning Point: Digital And AI Show Future Market Direction
Europe’s largest national in vitro diagnostics market is evolving post-COVID, under the effects of new digital laws, artificial intelligence-enabled devices, apps on prescription and the rising popularity of continuous glucose monitors. The VDGH industry association’s latest survey indicates a changing market landscape for IVDs in 2024.

J&J Refines Direction For Ottava
Delays have plagued J&J's soft tissue surgical robot. However, the company has now solidified at least its near-term plan and hopes to commence trials later this year.

'Blah-Blah' May Subside, But Harnessing AI Ethically, Impactfully Is Key For Medtech In 2024
Views from medtech industry leaders and investors on the most significant uncertainties and opportunities facing the sector in 2024.

Time Running Out To Comply With New EU Sustainability Legislation
At a webinar organized by the Association of the European Self-Care Industry, sustainability expert Onur Durmus, partner at ERM, gave an overview of the Corporate Sustainability Reporting Directive and what consumer health companies must do to ensure compliance.
Appointments
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Illumina Appoints Ankur Dhingra CFO
Ankur Dhingra has replaced Joydeep Goswami as Illumina’s chief financial officer after Goswami spent just 14 months on the job. The San Diego-based sequencing and array tech company also appointed Jakob Wedel as chief of strategy and corporate development officer.

Execs On The Move: February 2023
An interactive look at recent executive-level company changes and promotions in the medical device and diagnostics industries.

Execs On The Move: January 2024
An interactive look at recent executive-level company changes and promotions in the medical device and diagnostics industries.

News We’re Watching: LivaNova Names CEO; Surgical Robot Goes To Space, And More
Medtech Insight's News We're Watching highlights medtech industry developments we are following: LivaNova named a new CEO, Roche confirmed plans to launch a continuous glucose monitor, Virtual Incision sent its surgical robot into orbit, and Synchron announced a deal with Acquandas to advance its brain interface technology.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.