Clinical / R & D
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.
Clinical Trials
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.
Vivalink Partners With Rett Syndrome Research Trust To Accelerate Research In Rare Disease
Vivalink will provide ECG wearable technology to monitor patients in RSRT’s Vibrant study, which is aimed at assessing autonomic dysfunction in children with Rett syndrome.
Lilly Can Rest Easy As Tirzepatide Scores Phase III Sleep Apnea Win
Topline results from two studies in obstructive sleep apnea among obese adults showed efficacy crossing the 50% threshold that physicians have called clinically meaningful.

Clinical Trial Diversity Requires Sponsors Work With An Assortment Of Patient Advocates, Community Organizations
US FDA oncology officials are concerned that the entities sponsors are consulting in developing and implementing clinical trial diversity plans are not sufficiently diverse themselves and do not represent patients in underserved communities.

Interview: Theradaptive To Enter Clinical Trial For Spinal Fusion Implant
Theradaptive, which makes a device-biologic combination product to help speed bone healing, recently got authorization to start human trials from the US FDA. Medtech Insight spoke to CEO Luis Alvarez about the company’s past, present and future.
New Technology
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Vivalink Partners With Rett Syndrome Research Trust To Accelerate Research In Rare Disease
Vivalink will provide ECG wearable technology to monitor patients in RSRT’s Vibrant study, which is aimed at assessing autonomic dysfunction in children with Rett syndrome.

At-Home Health Testing Demand Is High Post-Pandemic, But So Are Barriers To Development And Use
At the recent Precision Med-Tri Con conference, laboratory experts traded views on the expansion of at-home testing for disease diagnosis and personalized health insights. While strong consumer demand spells opportunity, there are significant concerns about the accuracy and reliability of home-testing platforms, misuse, accessibility, and lack of health literacy.

MAISI: Navigating The 'Valley Of Death' In Medtech Research Translation
Translating research from proof of concept to clinical investigations is a difficult hurdle to overcome. To succeed, researchers need to design their technology for industrial standard manufacturing early on, Anne Vanhoestenberghe, director for the Manufacture of Active Implants and Surgical Instruments (MAISI), told Medtech Insight.

Medtronic Launches Remote Live-Stream Surgery, New AI Capabilities
Medtronic will launch a new feature that allows for live streaming of surgical procedures in more than 20 countries and the addition of 14 AI-driven algorithms to enhance surgical workflow.
Approvals
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.
Epitel Gets 510(k) Clearance On Remote EEG Technology
The REMI Remote EEG monitoring system and REMI Vigilenz AI could help patients with infrequent seizures get a quicker diagnosis.
Cochlear’s Osia System Receives Expanded FDA Clearance For Use In Younger Children
Kids ages 5 and up now can benefit from Cochlear’s Osia implant and sound processor, indicated for hearing loss, mixed hearing loss and single-sided sensorineural deafness.

News We’re Watching: AI Safety Partnership; Boston Scientific Recalls; New Cancer, STI Tests; VR
This week, the US and UK announced a partnership to promote AI safety. Boston Scientific recalls embolic agent. LumiCell received FDA approval for its imaging agent to detect residual cancer. Scout receives an award to develop an STI test; and Osso VR leverages the Apple Vision Pro for VR medical training.
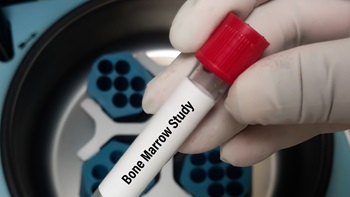
Israeli Company Lands De Novo Clearance For Digital Bone Marrow Application
The US FDA has cleared a novel software application to enhance the analysis of bone marrow smears. Using the AI-powered tool, hematopathologists may be able to better diagnose various blood and marrow diseases.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.