Swedish Company’s Novel Device At The Heart Of Milestone Transplant Surgery
Executive Summary
An organ preservation system from Swedish medical technology company XVIVO played a key role in the first successful transplant of a pig heart into a human patient.
When surgeons at the University of Maryland School of Medicine successfully implanted a donor heart into David Bennett, they had not only given hope to the 57-year-old-terminally ill patient, but to transplant candidates everywhere waiting to hear that a matching organ has been found.
What’s different about Bennett’s surgery, however, and why it brings such optimism, is that the donor heart that gave him a second chance didn’t come from another human, but a pig.
Pig hearts are used in cardiovascular research because their anatomical similarities to human hearts allow blood to flow in the same way it does in humans, which is also why pig heart tissue is used in making replacement valves for human hearts.
Still, prior attempts to use a pig’s heart in xenotransplantation – grafting or transplanting organs or tissues between different species – have failed because genetic differences cause the human body to reject the pig’s heart while viruses from the pig pose the risk of serious infection in the transplant patient.
Scientific advancements, however, have tackled this problem.
As XVIVO CEO Dag Andersson, whose heart perfusion device preserved the pig heart used in Bennett’s surgery, explained to Medtech Insight, pigs can be genetically modified to prevent immunological rejection from the human body.
“Recent advances in gene-editing tools are speeding up progress in this area,” Andersson said, noting that if a non-manipulated pig heart is used the human body will immediately experience hyperacute rejection, which is when the blood vessels clog and shut down.
“It was either die or do this transplant. I know it’s a shot in the dark, but it’s my choice.” – David Bennett
For Bennett’s surgery, three genes responsible for rapid antibody-mediated rejection were “knocked out” of the pig’s heart, according to a press release from the University of Maryland School of Medicine, while six human genes responsible for immune acceptance of the pig heart were inserted into its genome. Additionally, one gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, creating a total of 10 unique gene edits in the donor pig heart.
But as important as gene modification is to being able to successfully walk the high wire that is xenotransplantation surgery, the donor organ also needs to be properly stored and preserved.
The heart, Andersson noted, is the most challenging organ to preserve in a cold static state – the standard method – because the heart muscle is extremely sensitive to oxygen deficiency, and in heart transplant surgery the implanted heart needs to function immediately for the patient to live.
With cold static storage, Andersson said, the probability of long-term survival is diminished after 3 hours.
To address this, XVIVO developed a novel method for storing and transporting donated hearts through non-ischemic heart preservation (NIHP) in collaboration with Stig Steen, professor of cardiothoracic surgery at Lund University in Sweden and Swedish medical research company Igelösa Life Science. The XVIVO heart perfusion device stores the donor heart in a proprietary solution at 8 C˚ that is pumped through the heart during transport.
Though the company’s system is intended for clinical human to human transplants, Andersson said it has proved pivotal for long term survival in pre-clinical research using pig hearts for xenotransplantation by mitigating the risk of early organ dysfunction.
The US Food and Drug Administration granted the XVIVO heart technology “breakthrough device designation” in 2019.
“In a future where xenotransplants can help solve the donor organ shortage we are truly living our vision that nobody should die waiting for a new organ.” – Dag Andersson
While the Bennett surgery represents a “giant leap” forward in xenotransplantation, in Andersson’s view, it remains to be seen what it will mean for the medical procedure going forward. “The suggested next step will likely be a clinical trial to systematically study a population of patients transplanted with this method,” Andersson said.
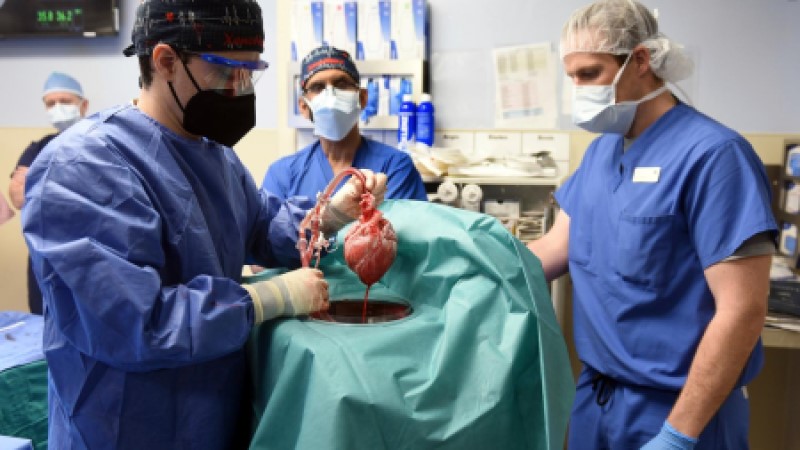 UNIVERSITY OF MARYLAND SURGICAL TEAM THAT PERFORMED THE FIRST SUCCESSFUL PIG HEART TRANSPLANT
University of Maryland
UNIVERSITY OF MARYLAND SURGICAL TEAM THAT PERFORMED THE FIRST SUCCESSFUL PIG HEART TRANSPLANT
University of Maryland
Xenotransplantation also holds the promise of solving the greatest challenge patients in need of transplant face – organ shortages. Only a small number of patients with end stage heart disease get the chance at a new heart while most die waiting.
But what is certain is that the surgery may have given a terminally ill patient out of options a new lease on life, according to Dr. Muhammad Mohiuddin, director of the Cardiac Xenotransplantation Program at the University of Maryland School of Medicine who was part of Bennett’s surgery team. “I am happy to say that his progress is on the track and he is in very good spirits. Without XVIVO’s new heart technology this transplant would never have happened,” Mohiuddin said.
“It was either die or do this transplant. I know it’s a shot in the dark, but it’s my choice,” said Bennett the day before his surgery.
The FDA granted emergency authorization for Bennett’s surgery, which took place on 7 January, through the agency’s expanded access, or compassionate use, provision. Bennett did not qualify for a human heart because of his condition.
As of 19 January, Bennett is still in the hospital but is making progress, according to Deborah Kotz, senior director of media relations at the University of Maryland School of Medicine, with no signs so far of organ rejection and the new heart beating on its own.
Bennett, Kotz told Medtech Insight, is no longer on ECMO (extracorporeal membrane oxygenation) – an advanced form of life support similar to a lung-bypass machine, which he was on for two months prior to undergoing the transplant procedure.
“He was on partial ECMO immediately after the surgery with his new transplant functioning at 50% capacity,” Kotz said, adding Bennett was taken off support entirely on 11 January and since then his new heart has been functioning at 100% capacity and working “like a rock star,” according to Dr. Bart Griffith, Bennett’s surgeon.
Kotz said Bennett will likely remain hospitalized for several weeks and if he continues to progress will go to a rehabilitation facility.
Meanwhile, Andersson remains hopeful.
“In a future where xenotransplants can help solve the donor organ shortage, we are truly living our vision that nobody should die waiting for a new organ,” he said.
