FDA Awash In Adverse Events: All-Time High Of 1.5 Million MDRs Reported In 2017
Executive Summary
US FDA statistics shared with Medtech Insight show that the number of Medical Device Reports sent to the agency dramatically rose last year, both in individual and summary formats. Might the agency's upcoming Voluntary Malfunction Summary Reporting Program slow the overall number of MDRs this year?
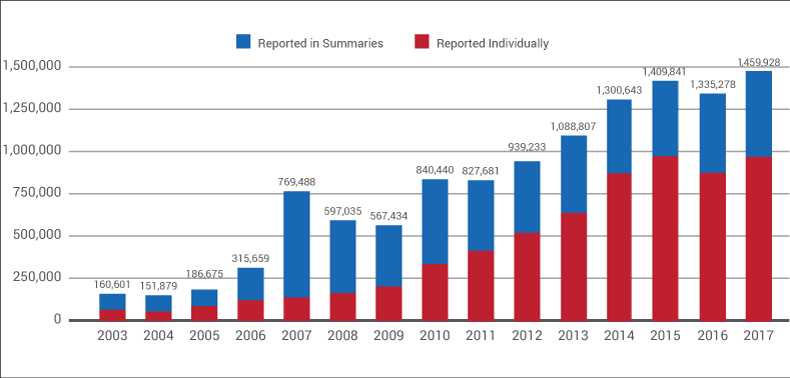
Device-users and companies had the dubious distinction of making history last year by sending the largest number of Medical Device Reports ever – nearly 1.5 million – to US FDA.
Statistics shared with Medtech Insight by the Office of Surveillance and Biometrics (OSB) within the agency's Center for Devices and Radiological Health show that 1,459,928 adverse events were submitted via the MDR system in calendar year 2017.
Adverse Events, 2003-2017
Source: US FDA
That represents a 9% increase from CY 2016, when 1,335,278 adverse events were submitted. (Also see "Adverse Events Dip Slightly In 2016; MDR Summary Reporting To US FDA Ticks Up" - Medtech Insight, 8 Feb, 2017.) Last year's figure is also 4% higher than the former all-time high of more than 1.4 million events, reported in 2015.
A total of 964,325 MDRs were sent to FDA on full MedWatch reporting forms in 2017. That's an 11% increase from 2016, when 871,654 individual reports were filed.
The remaining 495,603 reports were sent to FDA in 2017 as part of its Alternative Summary Reporting Program, which allows firms to submit abbreviated reports in a summarized, line-item format. That's up 6% from 2016's 465,624 summary reports – a modest increase that's headed in the anticipated direction, given the agency's recent work to boost the number of reports it receives in the form of summaries.
FDA announced in December that makers of an array of devices will soon be able to submit adverse events in a summary format to the agency through its upcoming Voluntary Malfunction Summary Reporting Program. (Also see "FDA Asks For More Summary Adverse Event Reports Under Proposed MDR Program" - Medtech Insight, 26 Dec, 2017.)
Under the proposed program – which does not extend to importers or user facilities – FDA is aiming to receive fewer individual MDRs. The program addresses goals outlined in a 2016 MDUFA IV commitment letter that directs the agency to allow makers of most devices to report adverse events quarterly in a summary format.
From the editors of The Gray Sheet