Q1 Recalls Snapshot: Device Corrections & Removals Are Down, But Recalls Of High-Risk Class I Units Remain A Concern
Executive Summary
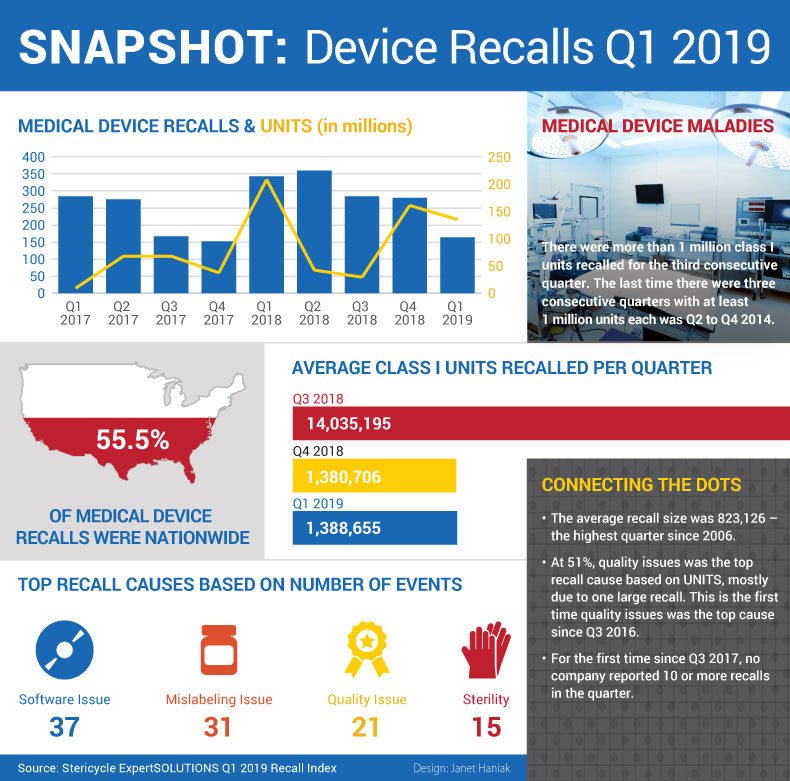
Corrections & removals of medical devices were down 41% in the first quarter of 2019, the lowest number of recalls recorded in a quarter since Q4 2017. There was one dark spot, though: Q1 was the third consecutive quarter in which more than 1 million high-risk class I units were recalled. Check out our Q1 recalls infographic.
Good news for industry: Corrections & removals of medical devices were down 41% in the first quarter of 2019, the lowest number of recalls recorded in a quarter since Q4 2017. There were 164 recalls in Q1, compared to the 280 noted in Q4 2018. And there were just under 135 million units recalled in Q1 (down 16% from Q4's 161 million), consulting firm Stericycle says in its most recent recalls index. However, there was one dark spot: It was the third consecutive quarter in which more than 1 million high-risk class I units were recalled. Meanwhile, problems with quality was the No. 1 reason for recalled units – the first time quality troubles was the top recall cause since Q3 2016.
Get a PDF of this infographic here.
