Q4 Recalls Snapshot: Recalled Device Units Soar 449%; Bad Software Leading Recall Cause For 11th Quarter In A Row
Executive Summary
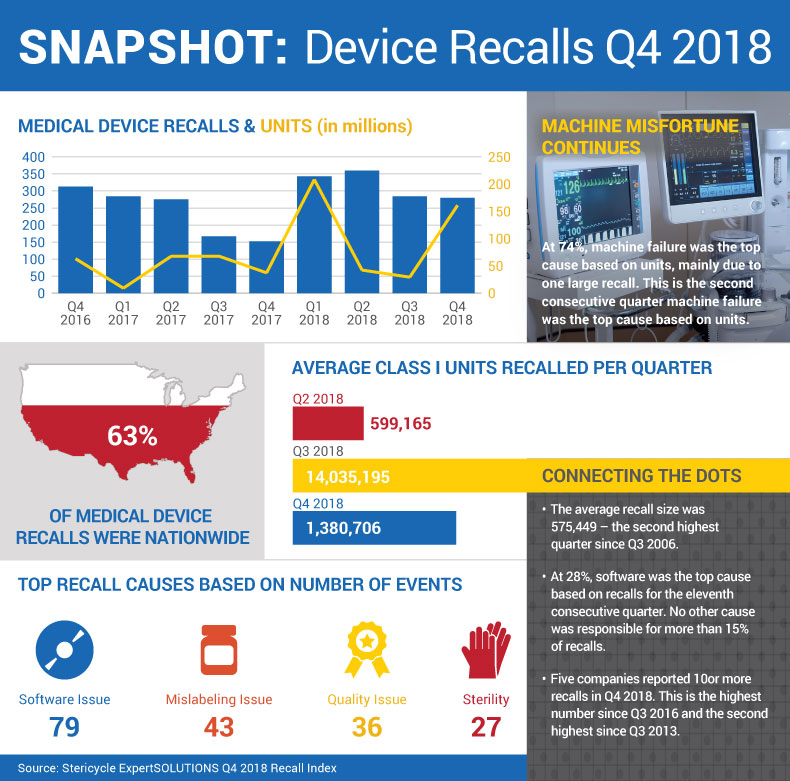
While the number of recalled products fell 1% in the fourth quarter of 2018, the number of recalled device units spiked dramatically. Machine failure was the top recall cause based on units, while software was blamed for causing the most recalled products for the 11th consecutive quarter. Check out our Q4 recalls infographic.
The fourth quarter of 2018 was a mixed bag for the medical device industry when it came to corrections & removals. While on the one hand, the number of recalled products remained relatively flat at 280 in Q4 (down 1% from Q3's 284 recalls), on the other, the number of recalled device units spiked dramatically. There were 161 million units recalled in Q4, an increase of 449% when compared to Q3's 29.4 million, consulting firm Stericycle says in its most recent recalls index. Machine failure was the top recall cause based on units – mostly due to one large recall – while software was blamed for causing the most recalled products for the 11th consecutive quarter. Meanwhile, five manufacturers initiated 10 or more recalls in Q4, the largest number of firms to do so since Q3 2016, and the second highest since Q3 2013.
Get a PDF of this infographic here.
From the editors of The Gray Sheet
