'Where Technology Meets Science': Doctors, Medical Students Take Experimental Medtech For A Spin In Pittsburgh's Center For Surgical Arts
Executive Summary
Some big-name device-makers – Medtronic, Stryker, Karl Storz, Siemens and more – are donating expensive capital equipment to the Center for Surgical Arts, a modern teaching lab that boasts 21 bays for training residents and fellows in various types of surgery. The companies are doing this to not only nail down future customers, but to test new devices, as well. The Center also includes a Bose test frame that was used to help clear Alphatec Spine's Solus device with US FDA without a clinical trial. "Those can be multimillion-dollar studies. So, to be able to do that, and to have the FDA feel comfortable with that, is kind of a cool capability," says Dr. Donald Whiting, chair of the Allegheny Health Network Neuroscience Institute.
Boyle Cheng zipped around the Center for Surgical Arts like a kid in a candy store, pointing out all his favorite gadgets – state-of-the-art medical devices he says make the surgical training facility second-to-none.
"Those pieces from Leica – the optics on those are world-famous. The quality is amazing," Cheng said, drawing attention to one of eight floor-mounted surgical microscopes that Leica Microsystems AG donated to the Center.
"And that piece over there is the O-Arm [from Medtronic PLC] – the CT-like quality you get from the imaging capability of this particular device is something that has helped tremendously in the OR," Cheng, director of research for the Allegheny Health Network (AHN) Neuroscience Institute, told Medtech Insight during a recent tour of the Center.
"And the C-Arm [made by Siemens AG] has been very, very helpful for orthopedic and neurosurgery procedures, too," Cheng said as he explained the various features on the device.
And there were many more medtech goodies – endoscopic towers from Karl Storz GMBH & Co., OR lights by Skytron, and robot-assist devices from Stryker Corp., just to name a few – stuffed inside the Center, which AHN opened in Pittsburgh's Allegheny General Hospital in mid-2016.
The modern teaching lab boasts 21 bays for training residents and fellows in neurosurgery, orthopedic surgery, cardiothoracic surgery, pain therapy interventions and interventional radiology, among other specialties.
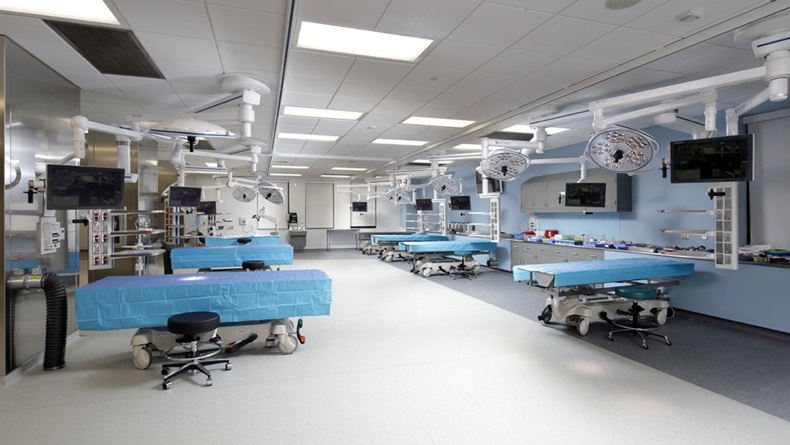
The Center "was meant to be a facility that could be used by all service lines. It's not just for neuroscience – it's for orthopedics and everybody else," said Cheng, who is also a professor at Drexel University's College of Medicine.
"Everyone in medicine benefits from understanding anatomy, surgical approaches, and ultimately how to treat patients," he said. "It's a facility open to anybody who wants to use it, and that includes [the device] industry, as well. We built the Center with the specific intent of medical education.
"The Center for Surgical Arts is really where technology meets science, and that's why it's an artform," Cheng added.
The Center includes some big-ticket hand-me-down devices from hospitals within AHN's network, including Allegheny General. But, like the Leica surgical scopes, many of the devices in the 7,400-square-foot Center were donated by manufacturers.
"There's a broad spectrum of device companies that are very much interested in a permanent showcasing" in the Center, Cheng said. "That latest Leica device, that's a piece of capital equipment that costs over $1m in the US – it's an amazing piece. Leica has been very generous."
AHN writes a grant proposal each time it wants to obtain a new (or used) piece of equipment from device-makers and hospitals.
"We wrote a grant specifically for the O-Arm – the mobile CT unit – and Medtronic agreed to let it undergo scientific review," Cheng said. "We told Medtronic that we wanted to train a lot of our residents and medical students that come through, and the firm said that scientifically that would be a great use of that capital equipment. It took a little bit of time – almost a year – before the process was finalized and we got the O-Arm."
"It's great marketing for the medical device manufacturers when they give their latest pieces of equipment to a training facility," AHN Neuroscience Institute's Boyle Cheng says.
The O-Arm used in the Center came from one of Allegheny General's own operating rooms and is the first generation of the device (there have been two generations manufactured thus far). Even though the O-Arm was already in the hospital that houses the Center for Surgical Arts, AHN still had to obtain a grant to move it from the OR to the Center's fourth-floor location in Allegheny General.
"Since a substantial value remained on the O-Arm, and the Center for Surgical Arts would benefit from the imaging capabilities, a grant submission was needed," Cheng explained.
Meanwhile, a stereotactic navigation unit the Center uses in conjunction with the O-Arm is seventh-generation.
Medtronic "just released the eighth generation of the navigation unit, so we're putting a grant in for that," Cheng said. "At $1m apiece, it can be difficult for [the company] to justify doing that, but we're hopeful they will."
He noted that AHN is in the midst of submitting yet another grant proposal to Medtronic, this time for a robot-assisted surgery device for the spine made by Mazor Robotics Ltd., which was acquired by Medtronic in September. (Also see "Medtronic Buys Mazor Robotics For $1.3Bn" - Medtech Insight, 21 Sep, 2018.)
"And, when you look at Stryker, they acquired the MAKO technology. Those are robot-assisted procedures. So, we're in discussions with them – writing grant proposals – to bring the latest robot-assisted technology into this space, as well," Cheng said.
Stryker bought MAKO Surgical Corp. in 2013. (Also see "Stryker bets big bucks on robotic surgery with MAKO buy" - Medtech Insight, 26 Sep, 2013.) The Center already has a few Stryker MAKO robot devices in its stable but is, as Cheng noted, looking to add more.
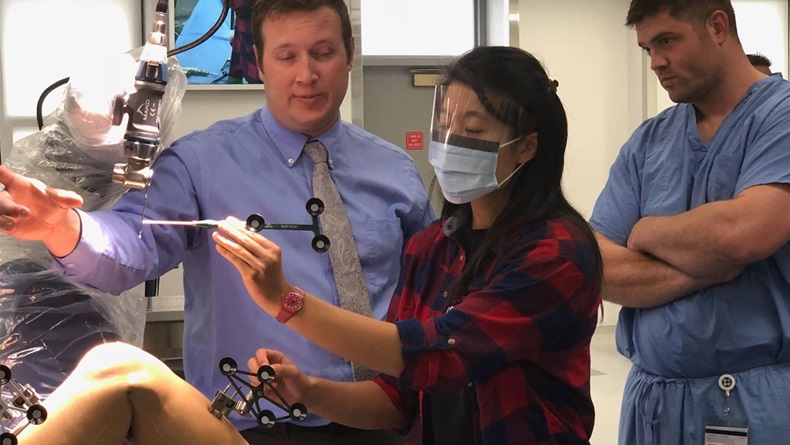
"It's great marketing for the medical device manufacturers when they give their latest pieces of equipment to a training facility," Cheng said. "And, when these young residents graduate and they start practicing, that means they've been trained on the latest technology, and they'll stick to the brand after."
That's one of the reasons why Medtronic donated its stereotactic navigation unit.
"At the time we applied for the grant [for the navigation unit], the firm said, 'This something that, when residents finish their studies, we want them to be familiar with. We want this technology not only at an academic center, but also at the community hospitals,'" Cheng said.
Medtronic can now say, "'Look how useful this equipment can be, not only for your training purposes, but in the OR and in the community hospitals,'" he added. "So, it's a great way to showcase a lot of the latest technology that comes out, and our residents benefit by understanding the cutting-edge technology."
Using AV For Training
The Center for Surgical Arts also boasts advanced audiovisual capabilities, enabling clinical instructors at a central station to educate and guide medical students, residents and fellows at the 21 individual work stations. The facility also allows for live-streaming of surgical cases from the hospital’s operating rooms into the teaching lab.
But those AV capabilities serve another purpose – remotely training doctors how to use the most up-to-date devices.
"Consider robotic surgery," AHN's Boyle Cheng said. "The robot-assisted pieces of capital equipment that are coming out, there's a high level of regulation, and as a part of that, in order to be compliant, [device companies] have to train the physicians that are using it, and the staff that are going to be eventually using that specific technology in the ER."
Without the training, "they can buy the devices, but they won't necessarily have the certification to use those pieces of equipment in the OR," he said. "So, instead of taking our physicians, taking the staff out of the practice, they're able to do that training right here [in the Center]. And if they're not able to join us, they can log onto an IP address and observe everything that's being pushed out."
It Isn't Just About Sales – It's About Testing New Devices, Too
But making money from potential customers is only one reason device-makers donate products that come with such a hefty price tag. The firms also use the Center to test new devices as they prepare for US FDA approval.
"The Center for Surgical Arts is regularly used by our team for training physicians in new techniques. Device companies come in and show us the new, cool tools they're developing. That also represents an opportunity for new companies that are developing, say, the next widgets, to bring their prototypes in and test them, and gain feedback," said Daniel Laurent, VP of internal and external communications for the Allegheny Health Network.
Device-makers "that are coming [to the Center] to train recent graduates or folks who are getting their training are oftentimes coming in with a brand-new FDA-approved device," Laurent said. The companies "want surgeons to be aware of the tool and how it's different from the tools they're already using."
But "in other cases we have developers bringing us a prototype that is not yet FDA-approved and they're trying to figure out how to optimize its performance, and we will test it," he said.
For example, doctors, residents and students in the Center are currently testing a new 3D component used with Synaptive Medical's MODUS V digital microscope.
"In surgery we do a lot of microscope work, and the MODUS V offers a big-screen TV and a robotically guided camera that follows the anatomy you want to address," Dr. Donald Whiting, chair of the AHN Neuroscience Institute, explained to Medtech Insight.
MODUS V reached the market in 2017.
"So basically, right now, the non-FDA-approved part is the 3D component," Whiting said.
The Center's MODUS V is 2D, "but we're working with the manufacturer on that 3D component, and we're also working with small instruments that go with that [3D capability] that will help identify normal tissue from abnormal – that is, whether [a mass of tissue] is a tumor," he said.
With the addition of 3D, "we'll be able to see that tumor on the monitor so we can use a 'paint-by-numbers' type of concept to take the brain tumor out," Whiting noted. "That's the real research part that we're starting to do with" Synaptive Medical.
Test Frame Leads To FDA Approval For Alphatec's Solus Device
The Center for Surgical Arts also developed a six-axis test frame in conjunction with Bose Corp. that mimics the pressure that can be applied to a spine.
Device firms that are testing new products "can place into it, say, prototype screws or implants, and the [test frame] puts that joint through stresses and strains that would simulate a year's, or five years', or ten years' worth of wear, and we can provide the developer with feedback on how their design is working in a much more expeditious fashion than if they were to wait a long period of time to get to that test," Laurent said.
Cheng said there are "a number of devices that are in development" that are using or plan to use the Bose test frame.
Manufacturers "will come to us and say, 'Hey, will this pass ASTM F1717?' or 'Will this pass the bone implant interface test under an ISO standard?'" Cheng said.
ISO is the International Organization for Standardization.
"All of the standards that are required by the FDA for clearance of a 510(k) device, the manufacturers will have to demonstrate to the FDA – and we have that capability with the test equipment we have in different labs throughout the facility," Cheng said.
While the Center's main lab is on the fourth floor, the large Bose test frame is housed in a separate seventh-floor lab.

In fact, when it was working toward FDA approval for its Solus anterior lumbar interbody fusion device, Alphatec Spine Inc. used the test frame in lieu of a clinical trial.
"What the FDA wanted to know was, would [Solus] stand up immediately postoperatively for the patient, meaning, when the patient got out of the OR, would they be OK in the short term? And the FDA really wanted to know, because [the Solus] device was the first of its kind, would it hold up to the loads that the human body would subject to this interbody device over the short term, immediately postop before the body heals? And that's what we were able to simulate," Cheng said.
"We were able to challenge the Solus device on our test frame, and Alphatec ultimately got approval for the device," he added.
That Solus received FDA approval without having to undergo a clinical trial is "pretty cool," AHN's Whiting said. "Those can be multimillion-dollar studies. So, to be able to do that, and to have the FDA feel comfortable with that, is kind of a cool capability."
From the editors of The Gray Sheet
