SSB Start-Ups
Executive Summary
Since its inception in 2010, the Singapore-Stanford Biodesign innovation training program has led to the filing of more than 22 patents and seven publicly funded projects. Of these seven projects, two have progressed to become start-ups – Advent Access and Privi Medical.
Since its inception in 2010, the Singapore-Stanford Biodesign innovation training program has led to the filing of more than 22 patents and seven publicly funded projects. Of these seven projects, two have progressed to become start-ups – Advent Access and Privi Medical.
Advent Access
Advent Access was founded and is now headed by Ruey Feng Peh, who was a Stanford Biodesign fellow and is currently program director for Singapore-Stanford Biodesign. The company's mission is to pioneer vascular access innovations to restore quality of life for kidney failure patients, and reduce the cost of dialysis in Asia and across the world.
According to Peh, the market for caring and managing kidney failure patients on hemodialysis is worth around $67bn today. However, up to a third of the costs of managing dialysis patients is not related to the dialysis technology itself, but to vascular access – specifically, access to a surgically created vessel, the arteriovenous fistula (AV fistula), which connects the artery and vein. It is this vascular access that allows the patient's blood to be removed, dialyzed and returned to the body.
The AV fistula is "a very precious, specially modified vein," said Peh, not only because it provides the lifeline for dialysis patients, but there are a limited number of sites where this fistula can be created.
However, for many years, the main way of accessing the AV fistula is by blindly sticking a needle into the patient in hopes of penetrating at the target spot. This inconsistent needling approach, which is highly operator-dependent, is not only painful for the patient, but exacerbates the wear and tear of the AV fistula. "These patients have to go through dialysis three times a week for generally four hours per session and they need two needles on their fistula each time. In literature, the average fistula lifespan is said to be about two to five years," said Peh. During this time, complications with the fistula can arise – a common one being aneurysms, another stenosis of the fistula – which require this vein to be repaired and ultimately be replaced by creating a new fistula, or switching to a synthetic AV graft, which has a shorter lifespan and a higher risk of complications.
"That's when the cost of maintaining these dialysis patients starts creeping up; when the fistula fails and you need to create another fistula or implant a graft through a repeat surgical procedure," Peh told Medtech Insight. "A lot of these complications could be preventable, as the frequent [and imprecise] needling cause weakness and injuries on the vein."
Many of the technologies in the area of vascular access for hemodialysis are looking at repair of the fistula, for example, angioplasty technologies, he said. "But not many are looking at preserving the fistula during its wear-and-tear phase. So Advent Access' technology is designed to delay the need for intervention and increase the longevity of the fistula." This will, he believes, ultimately lower costs and save the health-care payer money.
The av-Guardian by Advent Access comprises two implants made from medical grade titanium. These implants are designed to act as a "door" to the AV fistula. Each implant is placed subcutaneously on the "A" and "V" needle spots above the AV fistula, via a minimally invasive procedure. The implants are sutured to the skin for 14 days, after which the growth of tissue around the implants mean they should be "locked" into position naturally, and the sutures can be removed.
The av-Guardian system: stencil plates, implants and delivery device Source: Advent Access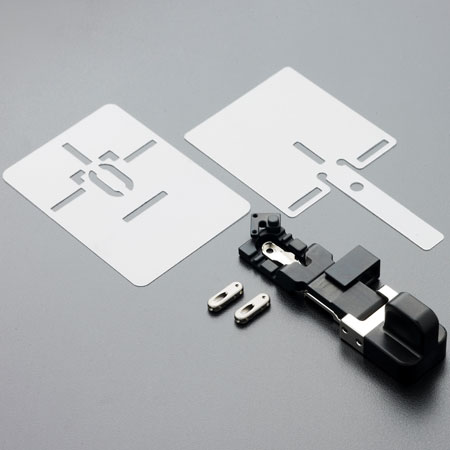
"We do a 'feel it, find it, follow it' technique. So the implant acts like a door that gives you access to the fistula. The caregiver will feel for the device [under the skin], have the needle penetrate the middle section of the device to find an inner lumen. Then you follow the lumen to guide the needle to the fistula," explained Peh. "So the needle will penetrate a specific part of the fistula, and the ability to penetrate the same spot of the fistula, at the same angle and at the same depth, allows a device-guided 'buttonhole track' to be formed over time."
This buttonhole track is effectively scarred-down tissue leading from under the skin to the fistula. When the track is formed, a blunt needle can then be used to access the fistula instead, resulting in less trauma to the AV fistula and less pain for the patient when they are being hooked up for dialysis. "It's akin to creating a piercing in your earlobe to wear an earring, like creating a high-quality and predictable 'earring track' with the help of the av-Guardian device," said Peh.
This buttonhole technique is not altogether new and has been around since the 1970s. Medical literature comparing the use of buttonhole tracks for regular vascular access with other needling techniques have demonstrated that the buttonhole technique, when executed properly, can reduce many of the common complications with failing AV fistulas and also delay intervention. However, one significant barrier has been the high level of skill required to master the buttonhole technique in order to yield these advantages reliably. The av-Guardian allows this technique to be improved and standardized so anyone can be a skilled nurse, said Peh. By removing this skill obstacle, this could, he believes, potentially allow dialysis to be performed by non-professional caregivers and even by the patients themselves, disrupting the traditional hemodialysis care delivery model and opening up the market for home and in-center self-dialysis.
"Home hemodialysis is an emerging technology and trend, but its penetration of the market is still only 1 percent. It is well known that vascular access is the Achilles' heel of dialysis and makes it difficult to be performed outside the [medical] center, so we are the last piece of the puzzle. For in-center dialysis, we learned that the top technical obstacle preventing centers from setting up self-care stations to minimize cost, or opening twilight shifts with minimal nurse support to treat a larger patient pool, is vascular access."
Advent Access is in the midst of a small clinical trial at Singapore General Hospital and National University Hospital to validate the safety of the av-Guardian. The company has also initiated efforts to apply for CE-mark approval. "Our experience in our clinical study [so far] has given us valuable learning, and we are looking at preparing our pipeline and platform to enable in-center self-dialysis, to help dialysis centers to treat more patients, at a lower cost, with their existing machines."
Peh added: “We are learning that what we have invented at Advent Access could not only impact vascular health, but also disrupt the way dialysis can be scaled to meet here-and-now needs of dialysis centers."
Privi Medical
Privi Medical, currently in stealth mode, was founded by three of the four fellows who graduated from SSB in 2014. During the fellowship program, Privi's co-founders – Prusothman Raja, Benjamin Tee and Rena Dharmawan – were charged with identifying a clinical need within the gastrointestinal space and developing a solution to meet this need.
Having gone through the clinical immersion that is integral to the SSB program, and then whittled down the hundreds of needs that they identified, Raja told Medtech Insight that the one clinical need that emerged at the end of the filtering process was for a solution to treat lower-grade hemorrhoids.
"Hemorrhoids is so common; you'd see a case of hemorrhoids at least twice a day in the gastrointestinal space," he said. But while there are clinical interventions available to treat more severe, grades 3-4 hemorrhoids, the choice of treatment options for grades 1-2 hemorrhoids is very poor. "We first observed this need in Stanford [Hospital, where we did part of the clinical immersion]. There, patients with grades 1-2 hemorrhoids were told to go home and nothing was given to the patient. They were just told to make some lifestyle changes – drink more water, eat better, try not to sit down for too long – and that's it."
With no real solution to relieve them of their symptoms, these patients' quality of life is significantly impacted. Additionally, the condition is a recurring one – sometimes as frequently as six to seven times a year in some cases, with each episode lasting one to two weeks – which further adds to the patient's distress.
The team therefore decided to develop a solution that could provide not only symptomatic relief, but also be drug-free so it addresses the concerns of pregnant women or new mothers, who make up a large proportion of hemorrhoid sufferers. "Some 80 percent of pregnant women develop haemorrhoids, and the patients we saw [during the clinical immersion] were new mothers. Most of them refuse to take any ointments or oral drugs for their hemorrhoids because they are worried about the effects of the active component in these products. So making our solution drug-free was among the early criteria we had set," said Raja.
Moreover, the team wanted to develop a device that can be used at home and self-administered by the patient. "The patients would be able to use this product to treat themselves at home without the doctor having to administer it. Also, with Asians [the incidence of hemorrhoids is three times higher in Asia – Ed.], they don't like going to the doctor repeatedly. The hemorrhoids bother them, but they would just tolerate it so they don't have to go to the doctor. So this is something that plays into our product," said Raja.
Being in stealth mode, the founders of Privi were not able to disclose the details of how their drug-free device works. However, Tee and Raja were able to tell Medtech Insight that it is a class I, single-use device, and the mechanism of action is one that is well known and, in scientific literature, has shown the ability to reduce symptoms of hemorrhoids.
Privi's technology has undergone proof-of-concept, and some animal and cadaver studies. The firm has to date received around S$0.5m of public and private funding, and is currently planning its first-in-man trial, with the possibility of gaining a CE mark and entering the market sometime in the second half of 2017.
From the editors of Clinica
